Foreword
New findings about the interaction between the immune system and the immune microenvironment have led to the development of important checkpoint inhibitors drugs for cancer. More specifically, these drugs target the PD-1/PD-L1 pathway.
One of these drugs, pembrolizumab, has been the subject of many articles and press releases.
It has even been hailed by some as the “syrup that dissolves tumors”, but this assertion needs to be clarified!
The press often goes beyond its own expertise by describing some treatments as miraculous but without having any knowledge of the drugs in question and without any actual rigorous documentation about the scientific studies published.
By doing this, the concerns of patients and their loved ones are not taken into account and we become caught in a vortex of words that do not contain any actual knowledge.
In view of this, we thought it would be useful to describe the main studies involving this therapy in our bi-annual review of the literature.
We will attempt to evaluate how this drug could be useful for the treatment of malignant pleural mesothelioma (MPM), and will describe as simply and graphically as possible some of the other immunotherapies that have been investigated or are being studied for MPM.
For the "insiders" who are involved in the study of MPM and for further information, please see the Reference section at the end of this revision for a list of publications.
Introduction
Immune checkpoint inhibitors
To prevent autoimmune phenomena, the activation and functions of T lymphocytes are finely regulated to different levels through “control points”, known as immune checkpoints.
Cytotoxic T lymphocytes associated with the antigen 4 (CTLA-4) and "programmed death 1" (PD-1) play an essential role in this process. Specifically, CTLA-4 (CD152) is an immunosuppressive receptor and a member of the superfamily of immunoglobulin CD28/B7, which is expressed mainly in the CD4 T lymphocytes and to a lesser extent in the antigen-presenting cells (APC) and granulocytes1 . The recruitment of CTLA-4 decreases the amplitude of the T cell response: it competes with its costimulatory CD28 receptor by inhibiting B7-1 (CD80) and B7-2 (CD86). These interactions are critically important for the initial activation of naïve T cells, by inhibiting the function of the T cells and preventing an inappropriate immune response against the “self” (or the body’s own) antigens in the secondary lymph organs and by limiting the extent and duration of the immune response2 .
On the other hand, the PD-1 pathway regulates the effector T cells in the later stages of the immune response in peripheral tissues3 .
PD-1 is expressed primarily in activated T and B cells, but also in monocytes, natural killer cells and tumor-infiltrating lymphocytes (TILS) 4 .
PD-1 binds two molecules, PD-L1 and PD-L2, which are members of the B7 family. PD-1 is expressed in leukocytes, while PD-2 only in dendritic cells and monocytes. PD-1 directly inhibits the functions of the TRC-mediated effector, acting as a negative regulator of the immune response. Additionally, PD-L1 is highly expressed in many malignancies, including MPM 5. PD-L1 expression in tumor cells appears to attenuate the immune response against tumors by inhibiting the activation of T cells and increasing the apoptosis of antigen-specific cell clones 6 7 .
CTLA-4 plays an important role in regulating the suppressive function of regulatory T lymphocytes (Treg), which are usually found in tumor tissue and are thought to locally inhibit antitumor immunity by inhibiting the response of effector T cells 8 9 .
In this context, by using a model of human suppressor T cell lines (MT-2), it has recently been shown that exposure to asbestos increases the function of Tregs with an increase in the production of suppressive cytokines such as IL-10 and TGFb 10.
In some tumors, the CTLA-4 and PD-1 inhibitory pathways are activated much more than in physiological conditions, so they are therefore believed to be involved in the suppression of the immune response by the tumor and in the ability to escape recognition by the immune system.
Various therapeutic approaches targeting CTLA-4 and PD-1/PD-L1 have therefore been designed over the last few years.
Anti CTLA-4
The efficacy of inhibiting CTLA-4 in combination with chemotherapy or radiotherapy in MPM has been shown in several in vivo and animal model studies 111213.
In fact, several mouse models of mesothelioma treated with an anti-CTLA-4 monoclonal antibody have demonstrated a significant inhibition of tumor growth, inhibition of repopulation by cancer cells, and an increase in T infiltrating lymphocytes 14.
Anti PD-1
PD-L1 expression has been reported in 40% of patients with mesothelioma and appears to be associated with a poor prognosis 15 16 17 . However, it is important to remember that guidelines and standard methods for investigating these targets and determining the cut-off, i.e, the thresholds indicating the positivity or negativity of this alteration, have not yet been defined 18. Another aspect still being investigated is clarifying which cell types should be evaluated for PD-L1 expression 19 20 .
Pembrolizumab
Pembrolizumab (MK-3475, lambrolizumab, Keytruda®) is a highly selective humanized IgG4 monoclonal antibody against PD-1. The amplified regulation of PD-1 occurs in some tumors and the pathway that is activated through this molecule helps to inhibit the active T cells as immune surveillance against tumors.
Pembrolizumab is able to destroy the bond that occurs between PD-1 and its PD-L1 and PD-L2 ligands, thus blocking the inhibitory signal to the T cells. By so doing, the cancer cells can be recognized by the cytotoxic T cells21 .
This drug was recently approved for solid tumors such as non-small cell lung cancer (NSCLC) expressing PD-L1, and advanced unresectable melanoma 22 23.
The diagram below shows the immune system activation against tumor cells and regulation of the signal occurring between PD-1 and its PD-L1-PD-L2 ligands.
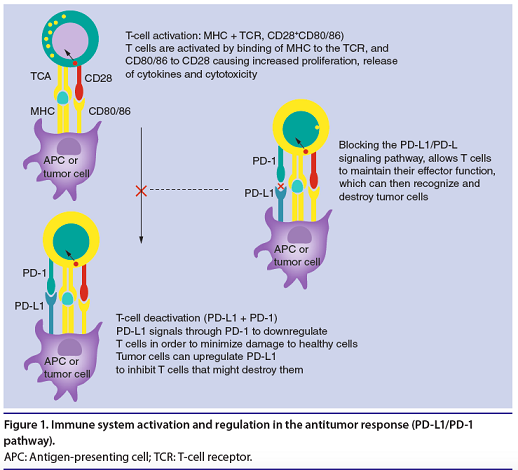
(From: S. Karim, et al. Future Oncol. 2016)
Efficacy of pembrolizumab
The efficacy of this drug has been recently documented in various clinical studies in patients with solid tumors of different histologies.
The Phase I KEYNOTE-001 study is evaluating the efficacy and safety of pembrolizumab in patients with NSCLC. The results of this study showed an objective response rate (ORR) of 19.4% with a median duration of response of 12.5 months, progression-free survival (PFS) of about 3.7 months, and a median overall survival (OS) of 12 months . An analysis of the results showed an association between the efficacy of pembrolizumab and the presence of PD-L1 expression. Specifically, in patients with expression of PD-L1>/= 50%, the ORR was 45.2%, together with greater PFS and OS rates versus those patients with lower PD -L1 expression25 .
Other studies have been initiated to assess the efficacy of the pembrolizumab, which for the sake of simplicity are listed in the following table:
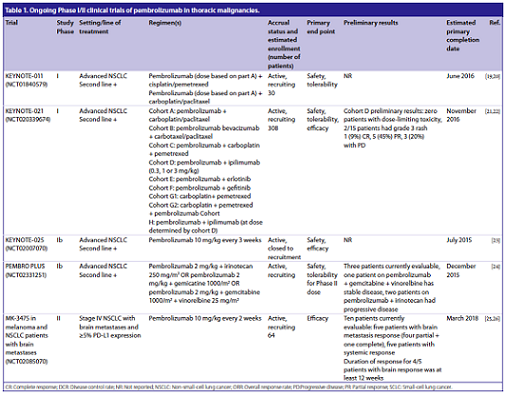
(From: S. Karim, et al. Future Oncol. 2016)
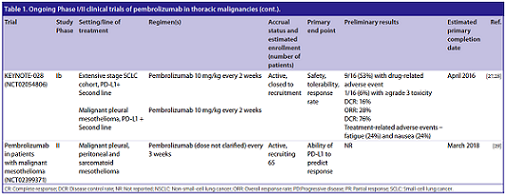
(From: S. Karim, et al. Future Oncol. 2016)
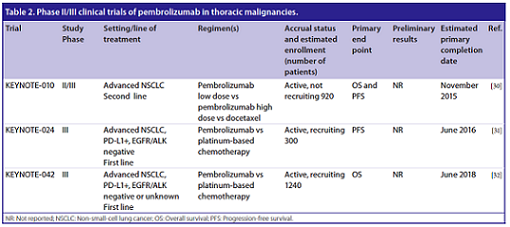
(From: S. Karim, et al. Future Oncol. 2016)
Safety of pembrolizumab
The safety and side effects of pembrolizumab were also evaluated in the above-mentioned KEYNOTE-001 study. We can say that pembrolizumab is generally well tolerated, without any clear evidence of adverse effects associated with it.
However, the most frequently reported side effects were asthenia, pruritus and decreased appetite.
The most serious grade 3 adverse events reported in a small percentage of patients (9.5%) were mainly dyspnea (3.8%), pneumonia (1.8%), decreased appetite (1%) and asthenia (1%) 26.
Some side effects involving the immune-mediated response were also reported. More specifically, there were cases of hypothyroidism (6.9%), pneumonia (3.6%) and drug infusion reactions (3%) 27.
Pembrolizumab and mesothelioma
The first data from the KEYNOTE-028 study of pembrolizumab in MPM were presented at the American Association for Cancer Research (AACR) conference.
Data from the study entitled "Single-agent pembrolizumab for patients with malignant pleural mesothelioma” were presented at the International Association for the Study of Lung Cancer (IASLC), World Conference on Lung Cancer (WCLC), in September 2015.
For completeness, the original abstract is included below:
“Single-agent pembrolizumab for patients with malignant pleural mesothelioma”
EW Alley, JH Schellens, A Santoro, K Beckey, SS Yuan, J Cheng, B Piperdi, LR Molife
Summary : Researchers presented updated safety and efficacy data for pembrolizumab (MK-3475), currently approved for the the treatment of advanced melanoma that progressed after ipilimumab, and BRAF inhibitor therapy if BRFV600 mutant in patients with PD-L1-positive advanced solid tumors with malignant pleural mesothelioma (MPM) enrolled in the KEYNOTE-028 study. In patients with PD-L1—positive MPM, pembrolizumab had significant clinical activity, they concluded. Further study of the durability of responses and the 49.4% 6-month progression-free survival rate is warranted.
Methods:
For this nonrandomized, multicohort phase 1b study, researchers included patients with measurable disease who had failed standard therapy, had ECOG PS 0-1, adequate organ function, and no autoimmune or interstitial lung disease.
They defined PD-L1 positivity as expression in ≥1% of tumor cells by IHC at a central laboratory.
Patients were treated with pembrolizumab (10 mg/kg) every 2 weeks for up to 2 years, or until confirmed progression or unacceptable toxicity.
Every 8 weeks for the first 6 months and every 12 weeks thereafter, researchers assessed response using RECIST v1.1.
The primary endpoint was overall response rate (ORR) and secondary endpoints included safety, tolerability, and progression free survival (PFS).
Results:
In all, 84 patients with MPM were screened for PD-L1 expression; of these, 38 (45%) had PD-L1—positive tumors; and of these, 25 were included in the study.
As of March 20, 2015, the ORR was 28% (n=7), and 12 patients (48%) had stable disease, for a disease control rate of 76%.
In 15 patients with only one previous line of therapy, ORR was 20% and DCR was 73%.
Researchers observed durable responses, with 10 (40%) of patients remaining on treatment.
After a median follow-up of 8.6 months, median PFS is 5.5 months (95% CI, 3.4-NR) and the 6-month PFS was 49.4%.
Researchers observed no new safety signals.
Drug-related adverse events (DRAE) occurred in 15 patients (60%), including 3 (12%) who had grade 3-4 DRAEs.
Only two patients required dose interruption due to immune-related adverse events, which included transaminitis and uveitis (1 each).
No treatment-related mortality occurred, and no patients discontinued treatment due to DRAEs.
The study design displayed below shows that pembrolizumab was administered to patients with advanced MPM who had progressed after prior treatment or who could not be treated with standard chemotherapy.
These patients had to be in overall good condition (ECOG PS 0-1) and PD-L1 positive. Patients with autoimmune diseases or interstitial lung disease were excluded.
Other baseline characteristics of the patients are summarized in the figure below:
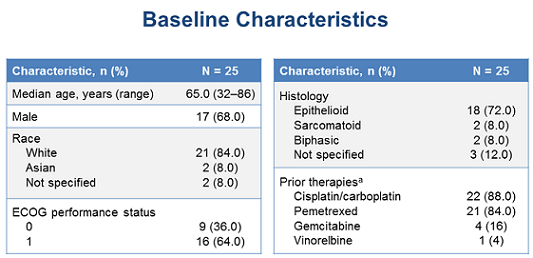
(From WCLC, September 7, 2015, Denver, Colorado, USA)
Patients received pembrolizumab intravenously at a dose of 10 mg/kg bi-weekly.
Response to treatment was assessed every eight weeks for the first six months and then every 12 weeks thereafter. If patients achieved a partial or complete response, they were treated for 24 months or until disease progression or the emergence of toxicity. Treatment with pembrolizumab was discontinued if patients experienced disease progression or unacceptable toxicity
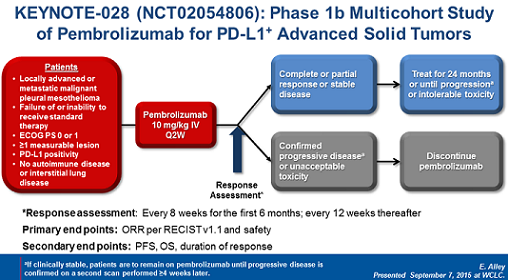
(From WCLC, September 7, 2015, Denver, Colorado, USA)
Below is an example of microscope images showing the negative or positive PD-L1 expression of this molecule.
(From WCLC, September 7, 2015, Denver, Colorado, USA)
Evaluated by immunohistochemistry, the PD-L1 levels do not appear to be correlated with the ability to respond better to treatment with pembrolizumab, as shown in this graph:
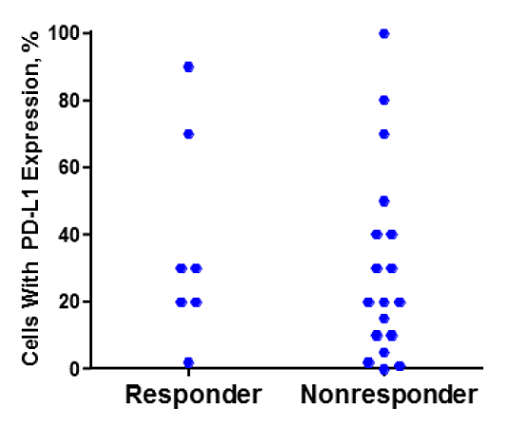
(From WCLC, September 7, 2015, Denver, Colorado, USA)
The drug was generally well tolerated, as evidenced by the low rate of serious side effects.
The most common adverse events were asthenia (24%) and nausea (24%); however, these side effects were reported in less than 20% of patients. Grade 3 side effects included an increase in transaminases (ALT) and thrombocytopenia. There were no patient discontinuations in the study due to pembrolizumab-related side effects, nor were there any drug-related deaths.
Below is a summary of the drug-related side effects:
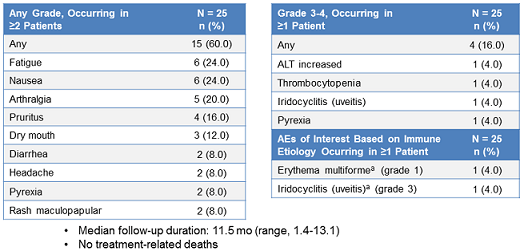
(From WCLC, September 7, 2015, Denver, Colorado, USA)
The anti-tumor activity of this treatment was as follows:
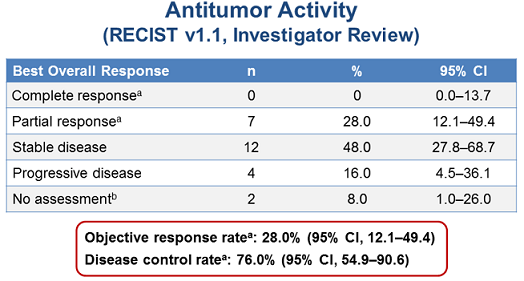
(From WCLC, September 7, 2015, Denver, Colorado, USA)
The PFS was approximately 5.8 months. Additionally, the PFS after six months of treatment was approximately 50%.
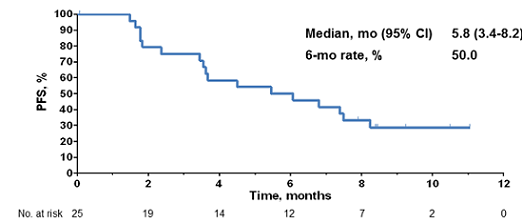
(From WCLC, September 7, 2015, Denver, Colorado, USA)
The results of this study are encouraging, but more studies are needed to confirm whether this treatment can actually increase the survival of these patients.
Other immunotherapy drugs
The Phase II study MEST-TREM-2008 (NCT01649024) is investigating tremelimumab, an anti-CTLA4 human monoclonal antibody, as monotherapy in patients with advanced MPM that has progressed after standard first-line chemotherapy. The results of this study can be summarized as follows: no patients achieved a complete response, while 31% had stable disease; the median overall survival was 10.7 months28 .
The Phase II study MEST-TREM-2012 (NCT01655888) evaluated a higher dose of tremelimumab, which led to the following results: a disease control rate of 52% was achieved, with a median overall survival of 10.9 months 29 30 .
An international, randomized, double-blind, placebo-controlled Phase IIb study is currently underway, DETERMINE (NCT01843374), in patients with advanced pleural or peritoneal mesothelioma who have progressed after a standard first-line therapy 31 .
Emerging immunotherapy findings have led to other study protocols, such as the Phase II study NIBIT-mESO-1 (NCT02588131), which is evaluating the efficacy of tremelimumab in combination with anti-PD-L1 durvalumab 32 .
The KEYNOTE-028 study (NCT02054806) investigated the efficacy of PD-1 antibody pembrolizumab in PD-L1-positive patients 33 34 .
The NCT02399371 study is also evaluating pembrolizumab in patients with MPM and the role of PD-L1 positivity 35 .
Additionally, pembrolizumab was recently investigated in combination with gemcitabine and defactinib (NCT02546531).
Another drug, nivolumab, was recently tested in MPM in the NivoMes study (NCT02497508), which enrolled patients with recurrent mesothelioma, and in a study investigating the combination of ipilimumab and nivolumab (NCT02716272), in patients with unresectable MPM 36 .
Avelumab is another PD-L1 inhibitor that was investigated for this disease, which resulted in a partial response of 15%, disease control rate of 60% and PFS of approximately 16.3 months 37 .
Future prospects
There are still many unresolved issues, and many aspects need to be clarified in order to better understand the results of these clinical trials. Of particular importance is the definition of a specific, sensitive biomarker to serve as a predictor of response because the association between PD-L1 expression and the response to immunotherapy remains controversial 39 40 .
It is important to remember, however, that there is no standardized method to test for this biomarker and a cut-off threshold to precisely determine positivity has not yet been defined 41.
In some MPM cases, the mutation burden was associated with an increase in the neo-antigen burden and inhibition of PD-1 42 43 .
Consequently, mutation burden and gene instability are aspects of the pathogenesis of MPM that need to be studied further in order to fully understand this disease and define the most effective drugs.
Although these immunotherapies appear to be very well tolerated, it should also be remembered that the toxicity of these drugs, particularly in the long-term, is not yet well known. Although rare, there may also be autoimmune side effects that could even be lethal 44.
Conclusions
In conclusion, the immune checkpoint blockade appears to be an attractive and certainly promising therapeutic strategy for the treatment of malignant pleural mesothelioma.
However, the results obtained to date are preliminary and many studies are still underway; as such, we are looking forward to the final results.
Additionally, drug combinations acting on different pathogenetic pathways related to immunotherapy must still be investigated.
The discovery of new, more sensitive markers that are predictive of response to these treatments will certainly be useful to identify patients who will truly benefit from these therapies.
References
1 E. Marcq, P. Pauwels, J.P. van Meerbeeck, E.L. Smits, Targeting immune checkpoints: new opportunity for mesothelioma treatment? Cancer Treat. Rev. 41 (10) (2015) 914–924.
2 S.L. Topalian, C.G. Drake, D.M. Pardoll, Immune checkpoint blockade: a common denominator approach to cancer therapy, Cancer Cell 27 (4) (2015)450–461.
3 E.I. Buchbinder, A. Desai, CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition, Am. J. Clin. Oncol. 39 (1) (2016) 98–106.
4 M.E. Keir, M.J. Butte, G.J. Freeman, A.H. Sharpe, PD-1 and its ligands in tolerance and immunity, Annu. Rev. Immunol. 26 (2008) 677–704.
5 A.S. Mansfield, A.C. Roden, T. Peikert, Y.M. Sheinin, S.M. Harrington, C.J. Krco, H. Dong, E.D. Kwon, B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis, J. Thorac. Oncol. 9 (7) (2014) 1036–1040.
6 J.A. Brown, D.M. Dorfman, F.R. Ma, E.L. Sullivan, O. Munoz, C.R. Wood, E.A. Greenfield, G.J. Freeman, Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production, J. Immunol. 170 (3) (2003) 1257–1266.
7 H. Dong, S.E. Strome, D.R. Salomao, H. Tamura, F. Hirano, D.B. Flies, P.C. Roche, J. Lu, G. Zhu, K. Tamada, V.A. Lennon, E. Celis, L. Chen, Tumor-associated B7- H1 promotes T-cell apoptosis: a potential mechanism of immune evasion, Nat. Med. 8 (8) (2002) 793–800.
8 K. Wing, Y. Onishi, P. Prieto-Martin, T. Yamaguchi, M. Miyara, Z. Fehervari, T. Nomura, S. Sakaguchi, CTLA-4 control over Foxp3+ regulatory T cell function, Science 322 (5899) (2008) 271–275.
9 K.S. Peggs, S.A. Quezada, C.A. Chambers, A.J. Korman, J.P. Allison, Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti CTLA-4 antibodies, J. Exp. Med. 206 (8) (2009) 1717–1725.
10 C. Ying, M. Maeda, Y. Nishimura, N. Kumagai-Takei, H. Hayashi, H. Matsuzaki, S. Lee, K. Yoshitome, S. Yamamoto, T. Hatayama, T. Otsuki, Enhancement of regulatory T cell-like suppressive function in MT-2 by long-term and lowdose exposure to asbestos, Toxicology 338 (2015) 86–94.
11 W.J. Lesterhuis, J. Salmons, A.K. Nowak, E.N. Rozali, A. Khong, I.M. Dick, J.A. Harken, B.W. Robinson, R.A. Lake, Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity, PLoS ONE 8 (4) (2013) e61895.
12 S. Demaria, S.C. Formenti, Radiation as an immunological adjuvant: current evidence on dose and fractionation, Front. Oncol. 2 (2012) 153.
13 L. Wu, M.O. Wu, L. De la Maza, Z. Yun, J. Yu, Y. Zhao, J. Cho, M. de Perrot, Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model, Oncotarget 6 (14) (2015) 12468–12480.
14 L. Wu, Z. Yun, T. Tagawa, K. Rey-McIntyre, M. de Perrot, CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma, Mol. Cancer Ther. 11 (8) (2012) 1809–1819.
15 A.S. Mansfield, A.C. Roden, T. Peikert, Y.M. Sheinin, S.M. Harrington, C.J. Krco, H. Dong, E.D. Kwon, B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis, J. Thorac. Oncol. 9 (7) (2014) 1036–1040.
16 C. Combaz-Lair, F. Galateau-Salle, A. McLeer-Florin, N. Le Stang, L. David- Boudet, M. Duruisseaux, G.R. Ferretti, E. Brambilla, S. Lebecque, S. Lantuejoul, Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas, Hum. Pathol. (2016).
17 Marcq E, Pauwels P, van Meerbeeck JP, et al. Targeting immune checkpoints: new opportunity for mesothelioma treatment? Cancer Treat Rev. 2015;41:914–924.
18 S.P. Patel, R. Kurzrock, PD-L1 expression as a predictive biomarker in cancer immunotherapy, Mol. Cancer Ther. 14 (4) (2015) 847–856.
19 A.M. Schultheis, A.H. Scheel, L. Ozretic, J. George, R.K. Thomas, T. Hagemann, T. Zander, J. Wolf, R. Buettner, PD-L1 expression in small cell neuroendocrine carcinomas, Eur. J. Cancer 51 (3) (2015) 421–426
. 20 R.S. Herbst, J.C. Soria, M. Kowanetz, G.D. Fine, O. Hamid, M.S. Gordon, J.A. Sosman, D.F. McDermott, J.D. Powderly, S.N. Gettinger, H.E. Kohrt, L. Horn, D. P. Lawrence, S. Rost, M. Leabman, Y. Xiao, A. Mokatrin, H. Koeppen, P.S. Hegde, I. Mellman, D.S. Chen, F.S. Hodi, Predictive correlates of response to the anti- PD-L1 antibody MPDL3280A in cancer patients, Nature 515 (7528) (2014) 563–567.
22 US Food and Drug Administration Press Release. www.fda.gov
23 US Food and Drug Administration website. www.accessdata.fda.gov
24 Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
25 Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
26 Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
27 Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
28 A. Hoos, R. Ibrahim, A. Korman, K. Abdallah, D. Berman, V. Shahabi, K. Chin, R. Canetta, R. Humphrey, Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy, Semin. Oncol. 37 (5) (2010) 533–546.
29 L. Calabro, A. Morra, E. Fonsatti, O. Cutaia, C. Fazio, D. Annesi, M. Lenoci, G. Amato, R. Danielli, M. Altomonte, D. Giannarelli, A.M. Di Giacomo, M. Maio, Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, singlearm, phase 2 study, Lancet Respir. Med. 3 (4) (2015) 301–309.
30 L. Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111.
31 Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308.
32 L. Calabro’, A. Morra, D. Giannarelli, D. Annesi, E. Bertocci, R. Danielli, M. Altomonte, A.M. Di Giacomo, M. Maio, Tremelimumab and Durvalumab combination for first and second-line treatment of mesothelioma patients: the NIBIT-MESO-1 study, in: 13th International Conference of the iMig Abstract Book, 2016, [abstract MS10.03]
33E.W. Alley, L.R. Molife, A. Santoro, K. Beckey, S. Yuan, J.D. Cheng, B. Piperdi, J. H.M. Shellens, Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: preliminary results from KEYNOTE-028, in: AACR Annual Meeting, 2015, [abstract CT103].
34 Alley EW, Schellens JHM, Santoro A, et al. Single-agent pembrolizumab for patients with malignant pleural mesothelioma. Presented at: 2015 World Conference on Lung Cancer; Sep 6–9; Denver, Colorado (US); 2015. Abs 3011.
35 J. Quispel-Janssen, M. Zimmerman, W. Buikhuisen, S. Burgers, G. Zago, P. Baas, Nivolumab in malignant pleural mesothelioma (NIVOMES): an interim analysis, in: 13th International Conference of the iMig Abstract Book, 2016, [abstract MS04.07]
36 J. Quispel-Janssen, M. Zimmerman, W. Buikhuisen, S. Burgers, G. Zago, P. Baas, Nivolumab in malignant pleural mesothelioma (NIVOMES): an interim analysis, in: 13th International Conference of the iMig Abstract Book, 2016, [abstract MS04.07]
37 Marcq E, Pauwels P, van Meerbeeck JP, et al. Targeting immune checkpoints: new opportunity for mesothelioma treatment? Cancer Treat Rev. 2015;41:914–924.
38 Marcq E, Pauwels P, van Meerbeeck JP, et al. Targeting immune checkpoints: new opportunity for mesothelioma treatment? Cancer Treat Rev. 2015;41:914–924.
39 Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
40 Alley EW, Schellens JHM, Santoro A, et al. Single-agent pembrolizumab for patients with malignant pleural mesothelioma. Presented at: 2015 World Conference on Lung Cancer; Sep 6–9; Denver, Colorado (US); 2015. Abs 3011.
41 Hassan R, Thomas A, Patel M, et al. Safety and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with advanced, unresectable mesothelioma: a phase IB trial. Presented at: 2015 European Cancer Congress; Sep 25–29; Vienna, Austria; 2015. Abs 3110.
42 McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469.
43 Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–416.
44 Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–574.
